Powerful Clinical Performance in International Patient Population
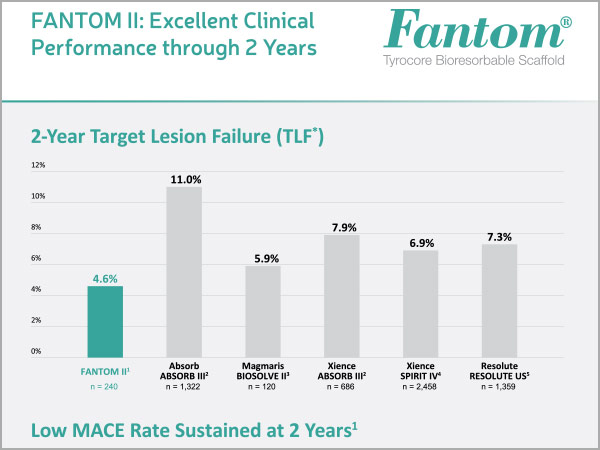
Excellent Clinical Performance through 2 Years
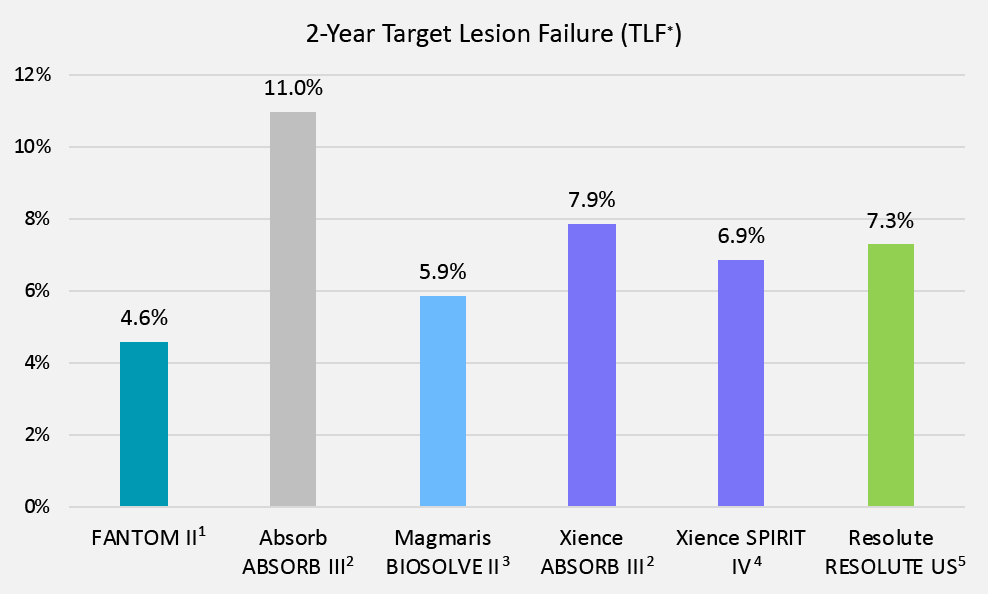
*TLF = cardiac death + target vessel MI + target lesion revascularization. The FANTOM II primary endpoint was Major Adverse Cardiac Events (MACE) = cardiac death + all MI + target lesion revascularization. The 24-month MACE rate was 5.0%.
FANTOM II Data Set
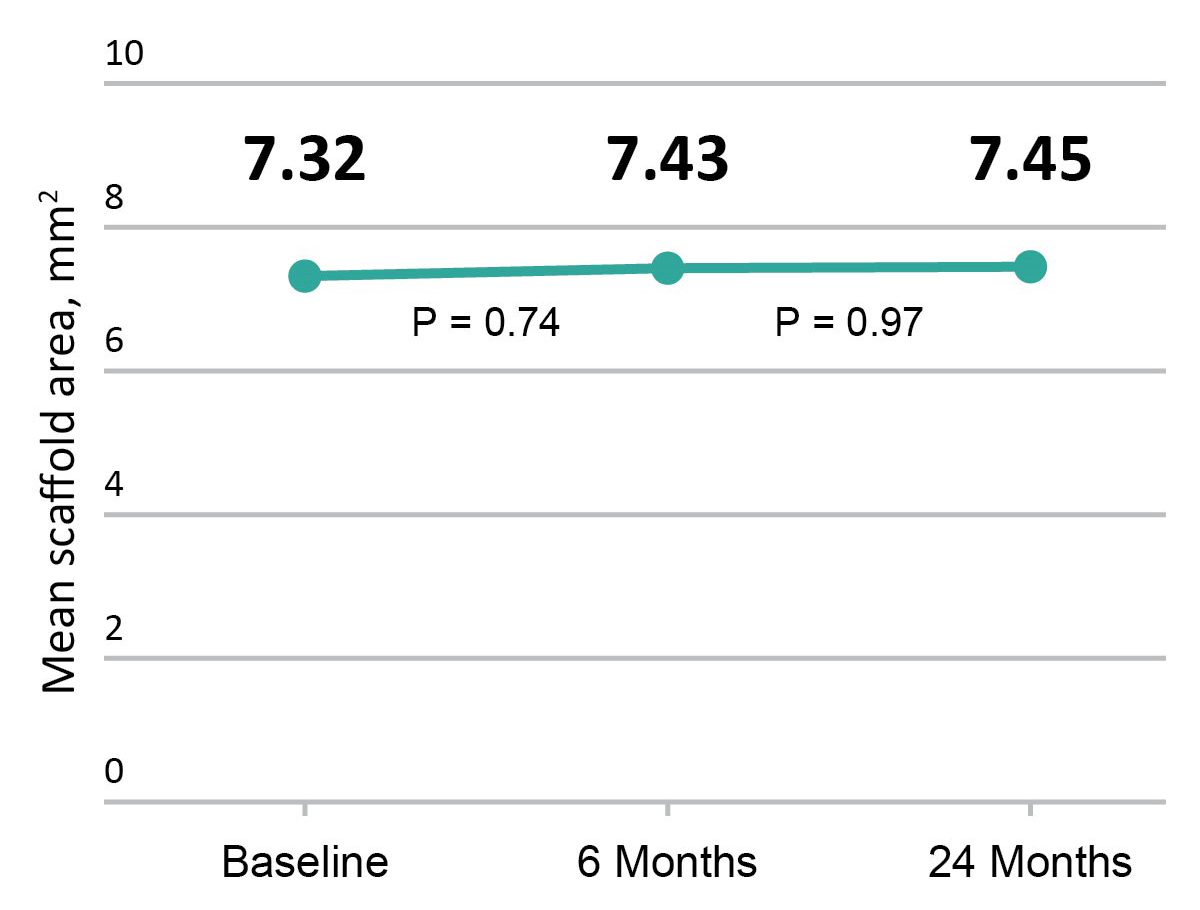
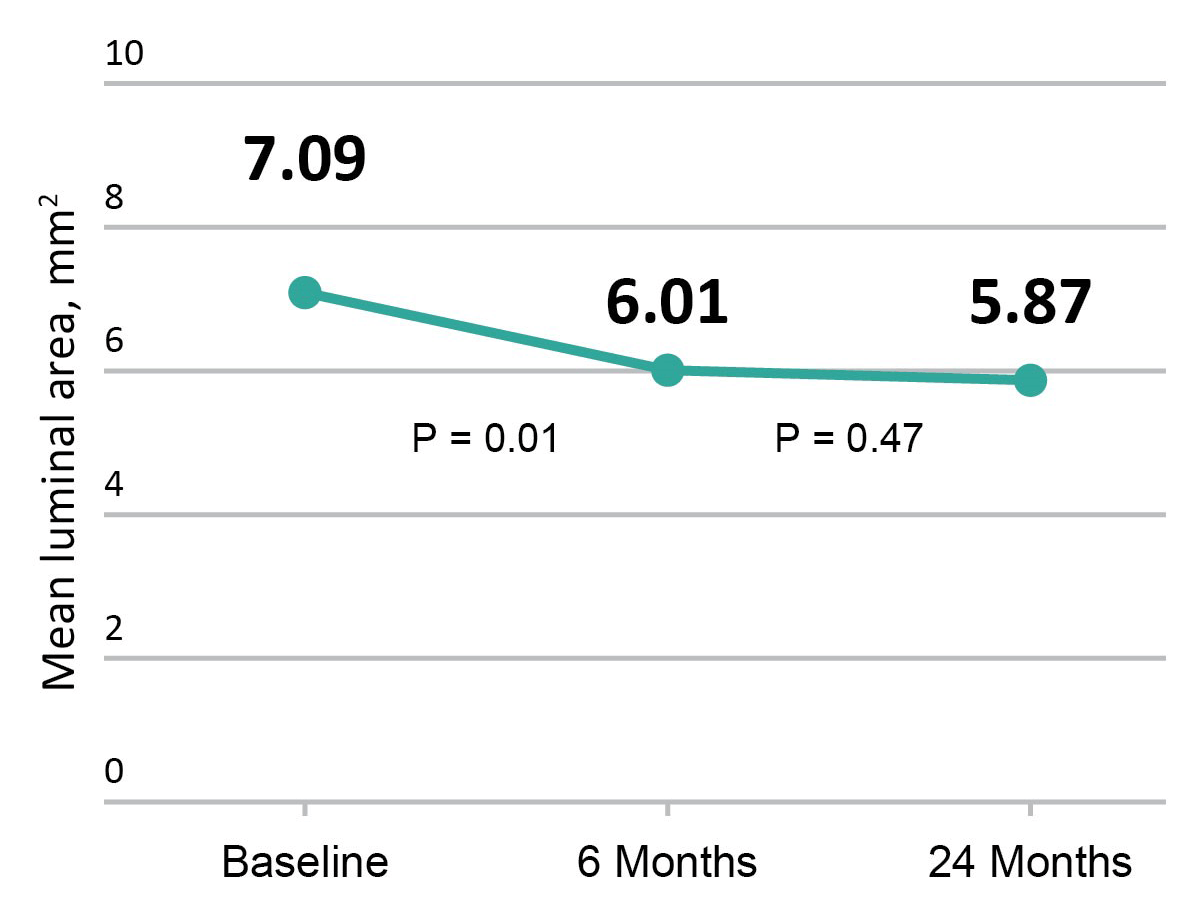
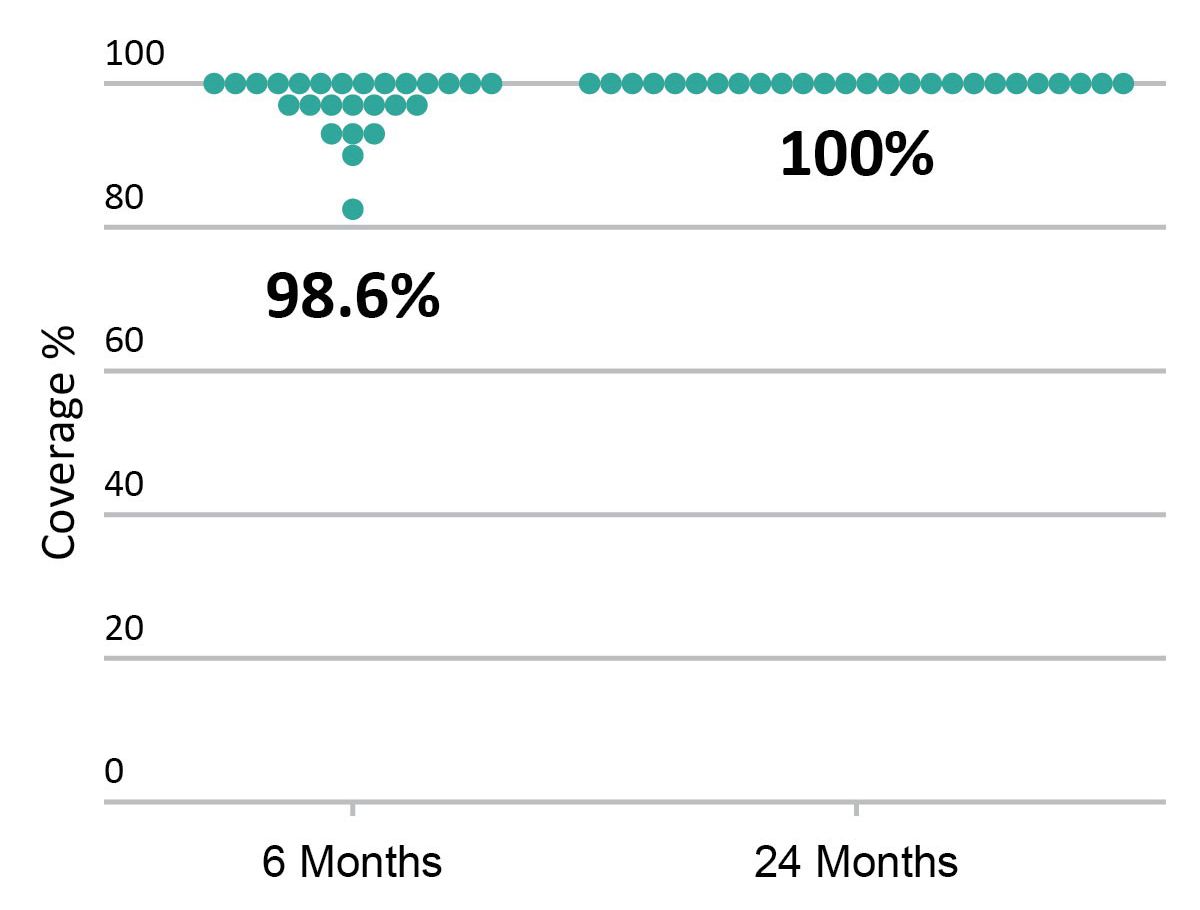
Excellent Vessel Healing & Scaffold Coverage
FANTOM II OCT analysis: matched OCT images at baseline, 6 months, and 24 months, n=256
Change in mean scaffold area
Change in mean luminal area
Strut coverage
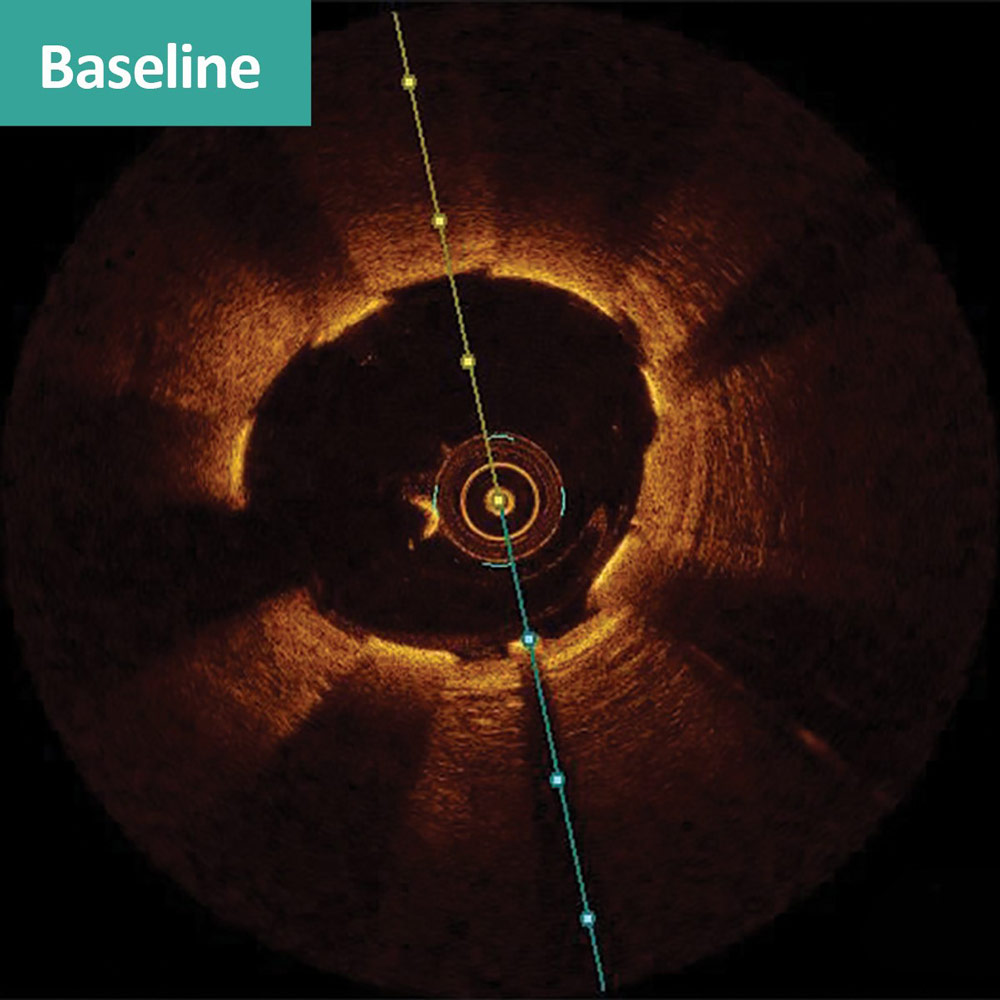
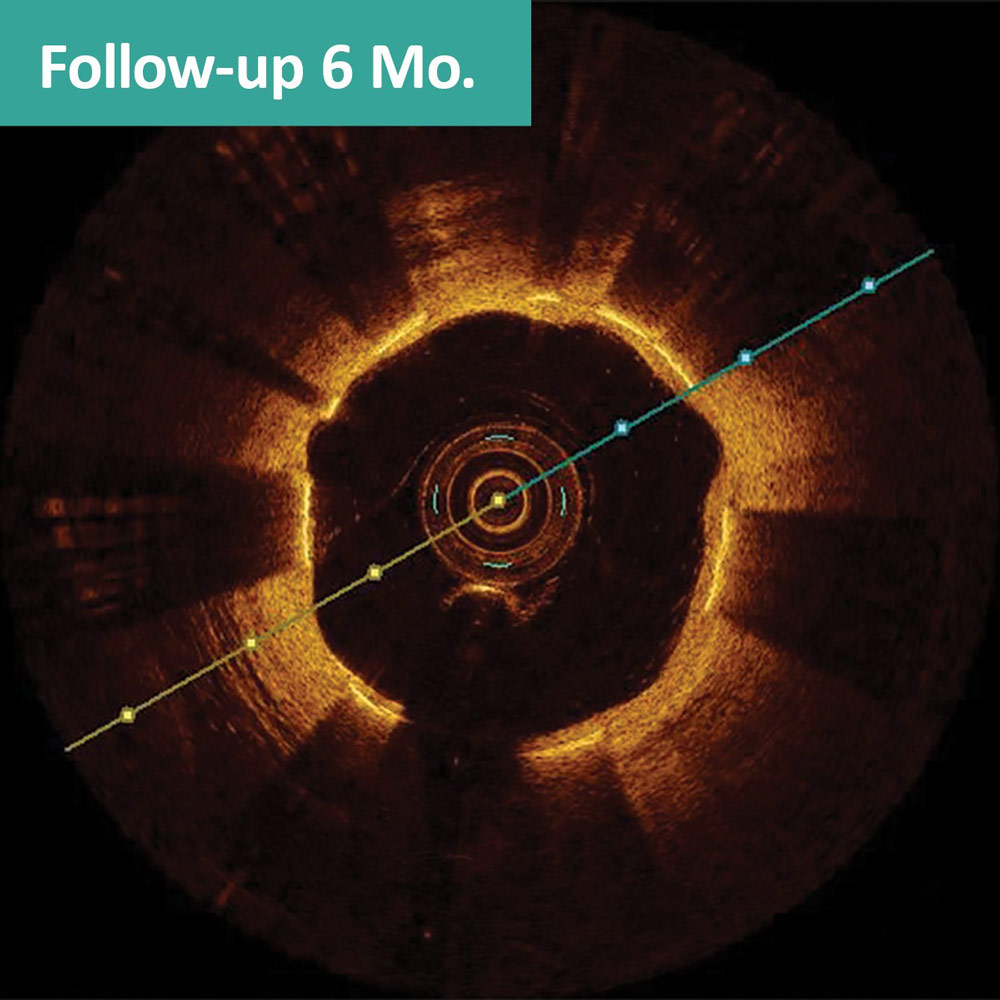
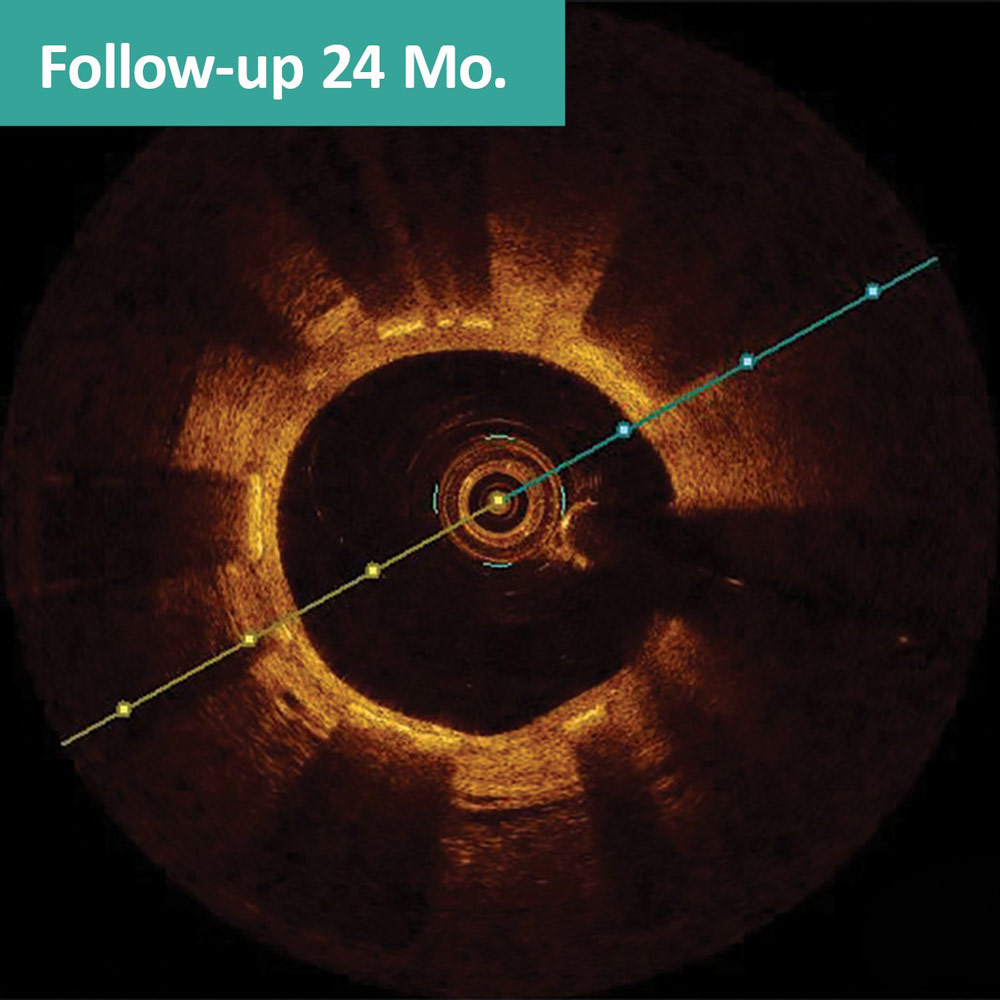
FANTOM II case sample: matched OCT images at baseline, 6 months, and 24 months1
FANTOM Global Clinical Program
REVA Medical is committed to advancing the field of BRS. The FANTOM Clinical Program is designed to evaluate performance in a variety of patient and lesion types.
Bibliography
FANTOM II 24-month Results, EuroPCR 2018.
FANTOM II 24-month OCT Analysis, EuroPCR 2018.
FANTOM II 12-month Results, EuroPCR 2017
FANTOM II 12-month OCT Analysis, EuroPCR 2017
FANTOM II Cohort A 6-mo Publication, JACC Cardiovascular Interventions 2017
Fantom Clinical Program, EuroPCR 2017
References:
- Abizaid, A. New 24-month data from the FANTOM II clinical trial. Presented EuroPCR 2018.
- Ellis S, Kereiakes, D. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent: ABSORB III. Presented ACC 2017.
- Haude M, et al. Short and midterm safety, clinical performance and multimodality imaging results of the drug-eluting absorbable metal scaffold: Combined data of the BIOSOLVE-II and BIOSOLVE-III trials. EuroPCR 2017.
- Stone G, et al. Randomized Comparison of Everolimus- and Paclitaxel-Eluting Stents 2-Year Follow-Up From the SPIRIT IV Trial. JACC 2011;58(1):19-25.
- Mauri L. 2-year clinical outcomes from the pivotal RESOLUTE US study. Presented ACC 2012.
- Holm, N. REVA Fantom II performance and healing patterns by OCT. REVA Symposium EuroPCR 2017.